Certifications and Compliance
ISO 27001 Certification
The Information Security Management System of SmartGene is certified in accordance with the requirements of the ISO/IEC 27001:2022 Standard.
The scope of the certification is for SmartGene's "Development and Operations of professional SaaS (Software as a Service) solutions for the analysis and the mangement of genetic data in health care and beyond." ISO/IEC 27001 is an international standard for information security, cybersecurity, and privacy protection management.
ISO 27001 Certificate number FR085987

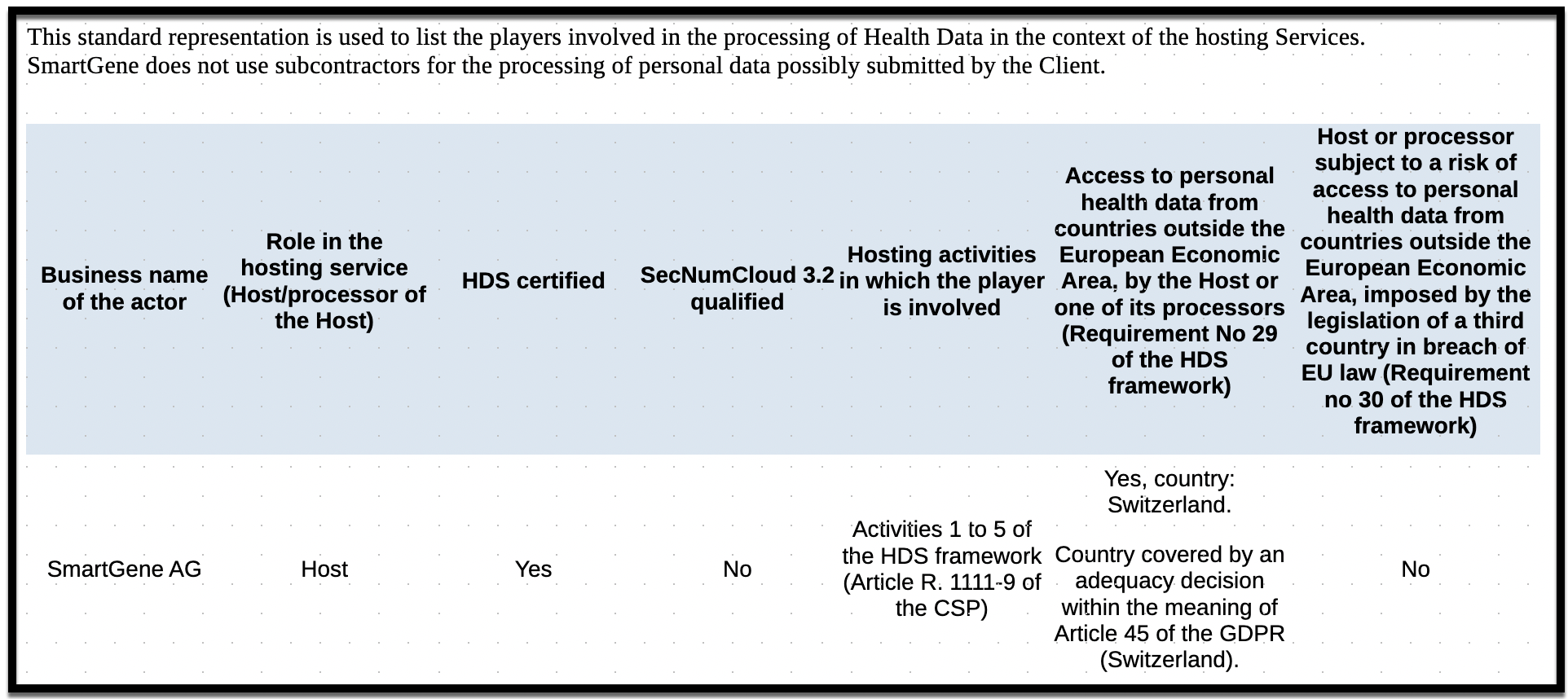
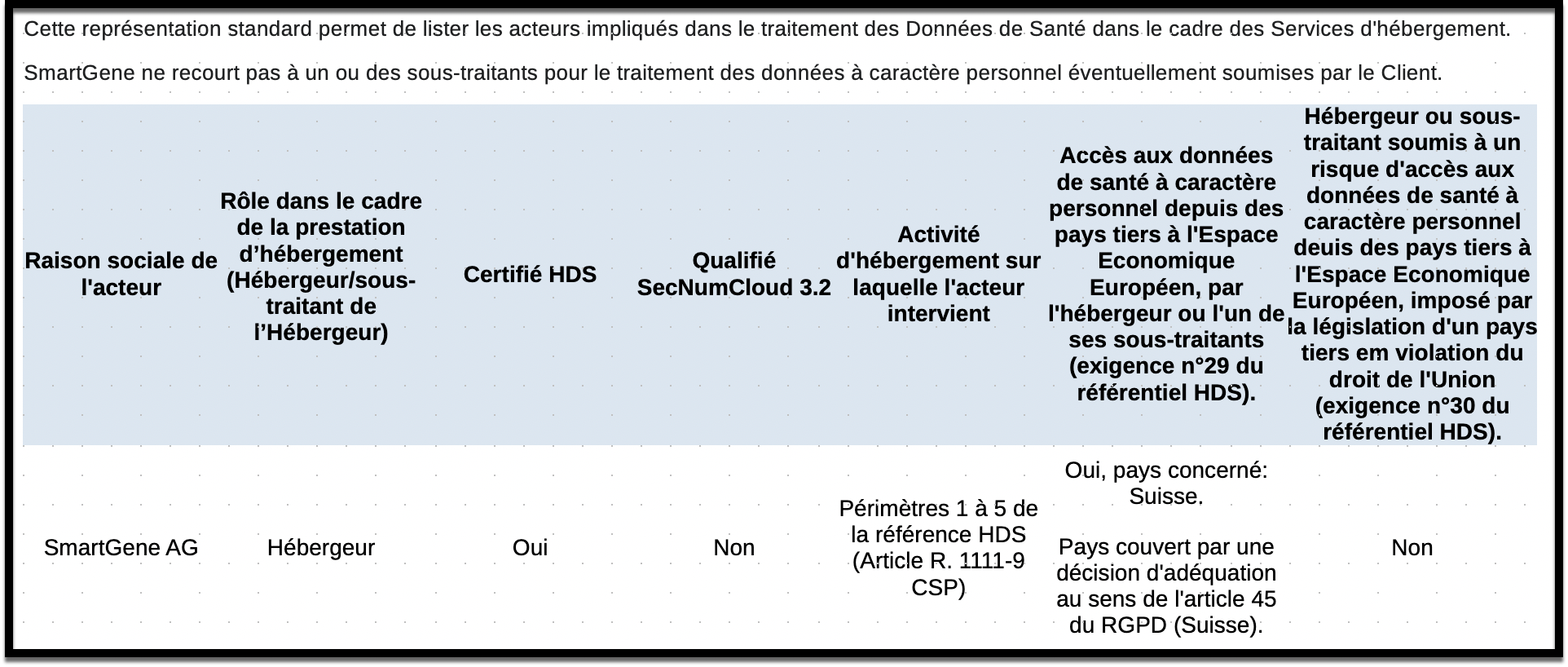
HDS Certification
SmartGene's Management System is in accordance with the requirements of the HDS Certification Referential version 2 - May 2024, related to the hosting of Health Data.
The scope of the certification includes the management and operation of the information system containing health data.
HDS Certificate number FR085864 (Health Data Hosting)

21 CFR Part 11
SmartGene is certified for the compatibility of its Integrated Database Network System® (IDNS) platforms and service apps under the requirements of 21 CFR Part 11.
This certification of compatibility under 21 CFR Part 11 is relevant for SmartGene’s customers who are operating under Good Manufacturing Practice regulations (GMPs).
CE-IVD
The SmartGene Service Apps for HIV-1, HCV, as well as for Bacteria and Fungi identification have obtained CE-IVD (In Vitro Diagnostic) labeling as notified to Swissmedic, the Swiss Agency for Therapeutic Products.
The aforementioned SmartGene Service Apps comply with the essential requirements of the IVD Directive 98/79/EC. SmartGene’s Quality Management System (QMS) provides all the technical documentation needed to assess this conformity, addressing software development, updates, risk analysis, and post-market surveillance.
Independently of the evolving regulatory landscape, SmartGene already applied Computer System Validation (CSV) guidelines for many years, as recommended or requested by regulatory authorities for medical device software.
